Molar Mass, Molecular Weight and Elemental Composition Calculator
Consider the following data for manganese atomic mass 54.938 electronegativity electron affinity ionization energy 717.3 heat of fusion 13.2 You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb e r (1) Mn (2) + 1 Mn(e) an't be decided with the data given.
Molar mass of Mn(NO3)2*4H2O is 251.0090 g/mol Convert between Mn(NO3)2*4H2O weight and moles
Elemental composition of Mn(NO3)2*4H2O
Formula in Hill system is H8MnN2O10 | |||||||||||||||||||||||||||||||||
Computing molar mass (molar weight)To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use:
Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Computing molecular weight (molecular mass)To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.Examples of molecular weight computations: C[14]O[16]2, S[34]O[16]2. Definitions of molecular mass, molecular weight, molar mass and molar weight
Give us feedback about your experience with Molecular Weight Calculator. Related: Molecular weights of amino acids | |||||||||||||||||||||||||||||||||
| molecular weights calculated today | |||||||||||||||||||||||||||||||||
- 54.938 g / mol is the atomic mass of Manganese.
- Relative atomic mass Mass percent (%) 55 Mn: 54.938050(1) 100: REDUCTION POTENTIALS; Balanced half-reaction E o / V; Mn IV + e.

Mn Average Atomic Mass
| Back to Online Chemical Tools Menu |
© 2021 webqc.org All rights reserved
| Periodic table |
| Unit converters |
| Chemistry tools |
| Chemical Forum |
| Chemistry FAQ |
| Constants |
| Symmetry |
| Chemistry links |
| Link to us |
| Contact us |
How to cite? |
WebQC.Org online education free homework help chemistry problems questions and answers |
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
Wireless keyboard and mouse for pc and mac. For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
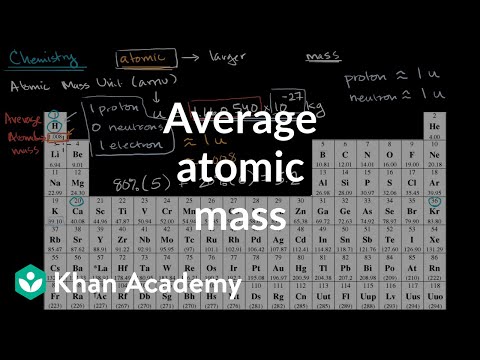
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
Mn Atomic Mass Number
Dead island for mac os. The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
