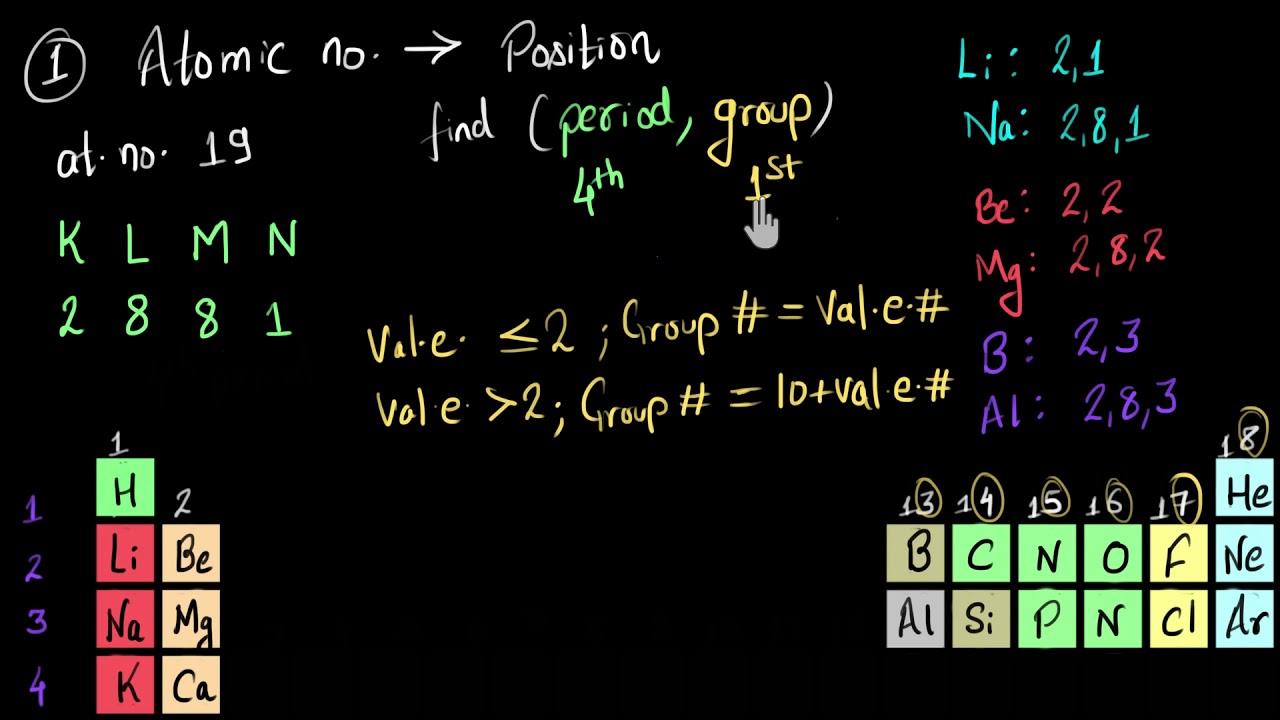
If an atom has an atomic number of 9 and an atomic mass number of 19, how many protons, neutrons, and electrons does it have?
1 Answer
This isotope of fluorine has 9 protons, 9 electrons and 10 neutrons.
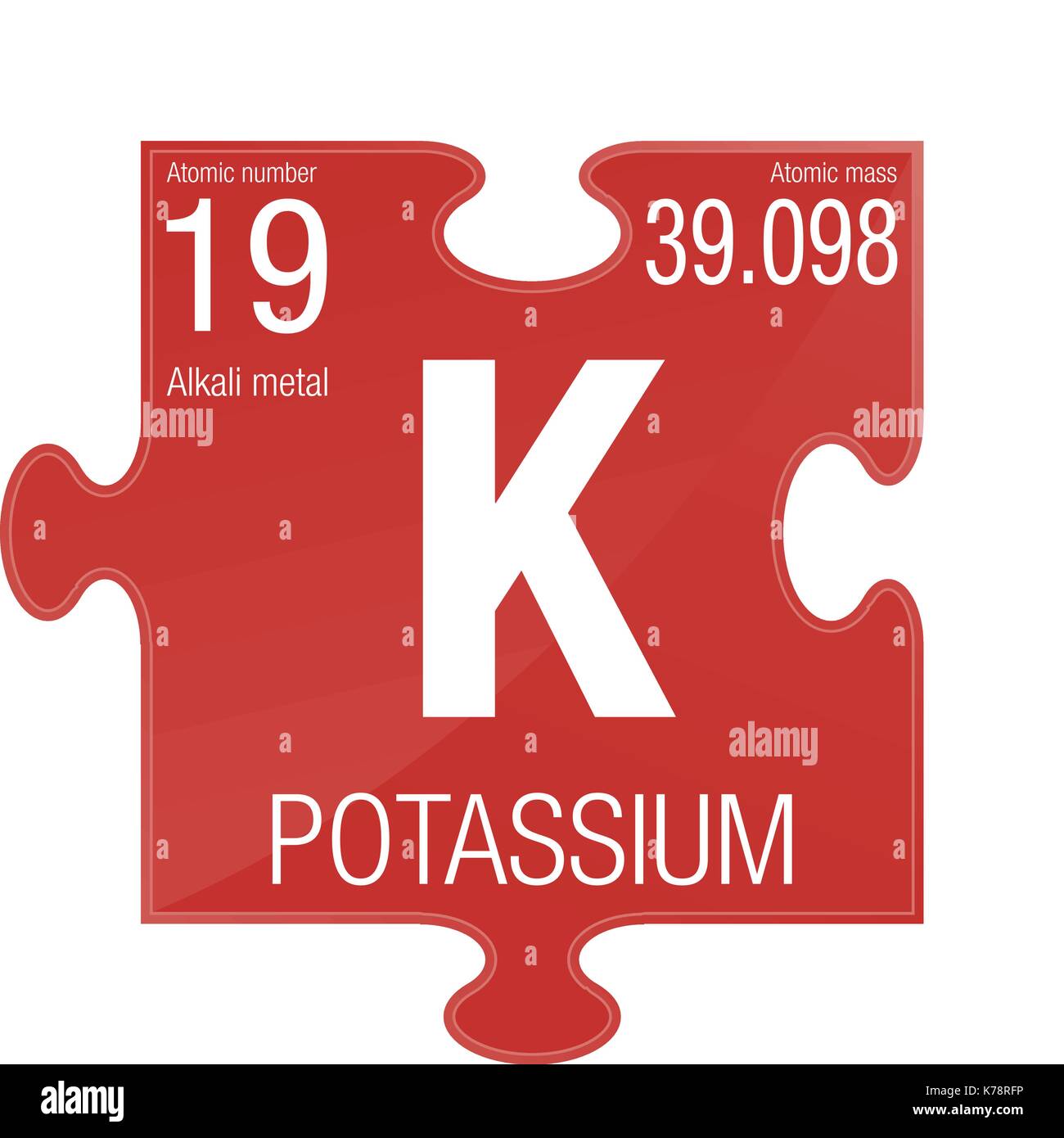
Potassium is element number 19. This means the atomic number of potassium is 19 and each. Potassium (K) is a silver-white coloured metal that has the atomic number 19 in the periodic table. It is an Alkali Metal and is located in Group 1 of the periodic table. It has the symbol K. Potassium is a highly reactive metal in Group 1 of the periodic table. Potassium like the other alkali metals needs to be stored in oil to prevent.
Explanation:
The atomic number is the number of protons
There are:
9 protons
9 electrons
10 neutrons
Fluorine is the element in question, as its atomic number is
The nuclear or isotopic notation would be
Related questions
Potassium is a soft, metallic-white, slightly bluish alkali metal that is naturally found bound to other elements in seawater and in many minerals. It oxidizes quickly on contact with air and reacts violently with water. It chemically resembles sodium.
In the periodic table, potassium is one of the alkali metals. All alkali metals have one valence electron in the outer shell, which is easily released to form a positively charged ion – a cation, which when combined with anions forms a salt. Potassium in nature is found only in ionic salts. Elemental potassium is a soft, silvery-white, alkaline metal that oxidizes rapidly in air and reacts violently with water, producing enough heat to ignite the hydrogen emitted in the reaction and burns with a purple flame. It is found dissolved in seawater (i.e. 0.04% potassium by weight), and is part of many minerals.
Potassium is chemically very similar to sodium, the preceding element in Group 1 of the periodic table. They have the same first ionization energy, which allows each atom to release its only outer electron. That they were different elements that combine with the same anions to make similar salts was suspected in 1702, and was proved in 1807 using electrolysis. Natural potassium consists of three isotopes, one of which, K is radioactive. Traces of K are found in all potassium, and it is the most common radioisotope in the human body.
Potassium ions are necessary for the function of all living cells. Transfer of K ions through nerve cell membranes is necessary for normal nerve transmission; Deficiency and excess of potassium can each result in many abnormalities, including abnormal heart rhythms and various electrocardiography abnormalities. Fresh fruit and vegetables are good sources of potassium. The body responds to the intake of dietary potassium, which increases serum potassium levels, by shifting potassium from outside into cells and increasing the excretion of potassium by the kidneys.
Read also: Foods Rich in Potassium (Kalium) | What Foods With Potassium in Them? Good For Heart And Bones
Most of the industrial applications, they exploit the high solubility of K compounds in water, such as K soap. Heavy crop production quickly depletes soil potassium, and this can be overcome with fertilizers containing potassium, which constitute 95% of global potassium production.
Health
Benefits
It is one of the most important minerals in the body. It helps regulate fluid balance, muscle contractions and nerve signals. What’s more, a high-potassium diet may help reduce blood pressure and water retention, protect against stroke and prevent osteoporosis and kidney stones.
They assist in a range of essential body functions, including:
Periodic Table Of Elements - Atomic Number, Atomic Mass ..
- blood pressure
- normal water balance
- muscle contractions
- nerve impulses
- digestion
- heart rhythm
- pH balance (acidity and alkalinity)
Deficiency
Burn for mac download. Certain conditions can cause deficiencies, or hypokalemia. These include:
- kidney disease
- overuse of diuretics
- excessive sweating, diarrhea, and vomiting
- magnesium deficiency
- use of antibiotics, such as carbenicillin and penicillin
The symptoms of hypokalemia are different depending on how severe your deficiency is.

A temporary decrease in potassium may not cause any symptoms. For example, if you sweat a lot from a hard workout, your potassium levels may normalize after eating a meal or drinking electrolytes before any damage is done.
However, severe deficiencies can be life-threatening. Signs of deficiency include:
- extreme fatigue
- muscle spasms, weakness, or cramping
- irregular heartbeat
- constipation, nausea, or vomiting
Atomic Number 19 Element
Hypokalemia is usually diagnosed with a blood test. Your doctor may also order an electrocardiogram of your heart and an arterial blood gas test to measure pH levels in your body.
Overdose
Too much of it can cause hyperkalemia. This is rare in people who eat balanced diets. Risk factors for overdose include:
- taking too many potassium supplements
- kidney disease
- prolonged exercise
- cocaine use
- potassium-conserving diuretics
- chemotherapy
- diabetes
- severe burns
The most obvious symptom of too much of it is an abnormal heartbeat (arrhythmia). Severe cases can lead to death. People with mild cases of high potassium rarely have noticeable symptoms. Your doctor should order occasional blood work if you have any risk factors.
Potassium in the periodic table
| Atomic number (Z) | 19 |
|---|---|
| Group | group 1: H and alkali metals |
| Period | period 4 |
| Block | s-block |
| Electron configuration | [Ar] 4s1 |
| Electrons per shell | 2, 8, 8, 1 |
Physical properties
| Physical properties | |
|---|---|
| Phase at STP | solid |
| Melting point | 336.7 K (63.5 °C, 146.3 °F) |
| Boiling point | 1032 K (759 °C, 1398 °F) |
| Density (near r.t.) | 0.89 g/cm3 |
| when liquid (at m.p.) | 0.828 g/cm3 |
| Critical point | 2223 K, 16 MPa |
| Heat of fusion | 2.33 kJ/mol |
| Heat of vaporization | 76.9 kJ/mol |
| Molar heat capacity | 29.6 J/(mol·K) |
Atomic properties

| Atomic properties | |
|---|---|
| Oxidation states | −1, +1 (a strongly basic oxide) |
| Electronegativity | Pauling scale: 0.82 |
| Ionization energies | |
| Atomic radius | empirical: 227 pm |
| Covalent radius | 203±12 pm |
| Van der Waals radius | 275 pm |
| Atomic radius, non-bonded (Å) | 2.75 | Covalent radius (Å) | 2.00 |
| Electron affinity (kJ mol−1) | 48.385 | Electronegativity (Pauling scale) | 0.82 |
| Ionisation energies (kJ mol−1) | 418.81 3051.83 4419.607 5876.92 7975.48 9590.6 11342.82 14943.65 | ||
Other properties
| Other properties | |
|---|---|
| Natural occurrence | primordial |
| Crystal structure | body-centered cubic (bcc) |
| Speed of sound thin rod | 2000 m/s (at 20 °C) |
| Thermal expansion | 83.3 µm/(m·K) (at 25 °C) |
| Thermal conductivity | 102.5 W/(m·K) |
| Electrical resistivity | 72 nΩ·m (at 20 °C) |
| Magnetic ordering | paramagnetic |
| Magnetic susceptibility | +20.8·10−6 cm3/mol (298 K) |
| Young’s modulus | 3.53 GPa |
| Shear modulus | 1.3 GPa |
| Bulk modulus | 3.1 GPa |
| Mohs hardness | 0.4 |
| Brinell hardness | 0.363 MPa |
| CAS Number | 7440-09-7 |
History
| History | |
|---|---|
| Discovery and first isolation | Humphry Davy (1807) |
Isotopes
Potassium (K) has 24 known isotopes with mass numbers varying between 32 and 55, as well as four nuclear isomers. K occurs in nature in the form of three isotopes: 39K (majority) and 41K, both stable, and a long-lived radioisotope (half-life of 1.248 billion years), 40K. Its standard atomic mass is 39.0983 (1) u. The other potassium radioisotopes all have a half-life of less than one day, and most of them less than one minute.
| Main isotopes | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
Periodic Table of Elements | Complete List of Chemical Elements by Group, Name, Symbol, Color and Type
Atomic Number 197
Sources: Royal Society of Chemistry,
Chemical Elements.com - Potassium (K)
Photo credit: Wikimedia Commons
Atomic Number 19 Mass Number 41
Justfocus. Photo explanations: pieces of potassium metal. Cut Potassium pieces from Dennis s.k collection.
